Abstract
Background: Although in recent clinical trials the 5-year event-free survival rates for pediatric Acute Myeloid Leukemia (AML) range between 49% and 64%, relapse still occurs in up to one-third of cases. Long-term outcomes of children with relapsed or refractory disease remain poor, with overall survival under 40%. Novel drugs directed toward distinct pathways and new molecular targets are required to continue improving outcomes for this aggressive hematological disease.
Use of the BCL-2 inhibitor, venetoclax, has improved overall and event-free survival in adult patients with newly diagnosed, intensive-chemotherapy ineligible AML, however, its effects in pediatric AML patients remain unclear. We reviewed our multi-institutional experience with venetoclax in this population.
Methods: We performed a retrospective review of patients ages 0 to 23 y/o with AML and treated with at least one cycle of venetoclax at either MD Anderson Cancer Center or Texas Children's Hospital prior to May 2021. Flow cytometry was used to assess morphology and response to therapy. Adverse events (AEs) associated with venetoclax were graded according to the Common Terminology Criteria for Adverse Events version 5.0. Descriptive statistics were used to report efficacy and toxicity data.
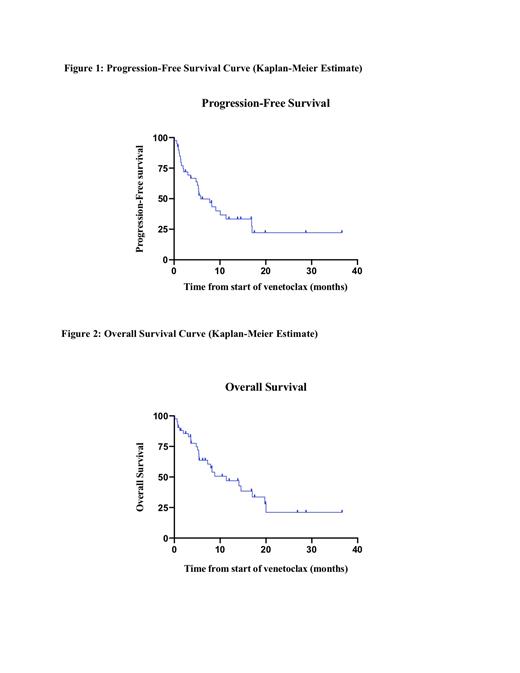
Results: 46 patients with AML who received venetoclax-containing regimens were identified. There was a similar proportion of males and females (52% and 48%) and most were Caucasian (48%). The median age was 21 years (range, 1-23) with eight patients (17%) <12 years of age. The most common AML subtype was M2 (30%; n=14) and the most common genetic mutations were FLT3-ITD (15%; n=7) and KMT2A (26%; n=12). Eight (17%) patients received venetoclax within front-line therapy and 38 (83%) as part of therapy for relapsed or refractory disease. Patients received a median of 2 therapies (range, 0-7) before venetoclax, with ten patients (22%) receiving at least four lines of prior therapy and twenty (43%) at least one prior allogeneic transplant. Venetoclax was combined with a hypomethylating agent in 28 (61%) patients. Dosing varied significantly ranging from 34-479 mg/m2 with 20% (n=9) requiring dose reduction due to concomitant use of azole antifungals. The most common grade 3/4 AE's observed included febrile neutropenia (41%; n=19), neutropenia (35%; n=16), anemia (20%; n=9), and thrombocytopenia (33%; n=15). No patients discontinued treatment because of AEs. The overall response rate in the 38 patients evaluated for disease at the end of cycle 1 was 53% (n=20). Complete remission (CR) or complete remission with incomplete blood count recovery (CRi) was achieved in 42% (n=16) of patients. Among those with CR/CRi, 9 (56%) were MRD negative. Of those sixteen, 4 (25%) had received venetoclax as front-line therapy. In total, seventeen patients (37%) were able to successfully transition to hematopoietic stem cell transplant. The median follow-up time was 8.5 months. Median progression-free survival (PFS) was 5.5 months (range, 0.13-36.61) and overall survival (OS) was 10 months (range, 0.13-36.61). Patients who received venetoclax as part of front-line therapy had a median PFS of 16.8 months (range, 0.72-17.5) and OS of 16.8 months (range, 0.72-28.75) with 4 (50%) patients still in ongoing remission.
Conclusion: Our findings suggest that in pediatric and early young adults, venetoclax is well-tolerated with a variety of cytotoxic agents and has a safety profile similar to that in adults. Patients should be monitored closely for prolonged myelosuppression and febrile neutropenia. As dosing varied significantly in our cohort, more studies are needed to establish an optimal dose in the pediatric population. Longer follow-up is expected to provide more insight on the improvement venetoclax may have on overall and progression-free survival.
Kadia: Sanofi-Aventis: Consultancy; Dalichi Sankyo: Consultancy; Genentech: Consultancy, Other: Grant/research support; Genfleet: Other; AstraZeneca: Other; Astellas: Other; Pulmotech: Other; Cure: Speakers Bureau; Cellonkos: Other; Jazz: Consultancy; Pfizer: Consultancy, Other; Ascentage: Other; Novartis: Consultancy; Liberum: Consultancy; BMS: Other: Grant/research support; Amgen: Other: Grant/research support; Aglos: Consultancy; AbbVie: Consultancy, Other: Grant/research support. Konopleva: Cellectis: Other: grant support; F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; KisoJi: Research Funding; Calithera: Other: grant support, Research Funding; Rafael Pharmaceuticals: Other: grant support, Research Funding; Ascentage: Other: grant support, Research Funding; Sanofi: Other: grant support, Research Funding; Forty Seven: Other: grant support, Research Funding; Ablynx: Other: grant support, Research Funding; Agios: Other: grant support, Research Funding; AstraZeneca: Other: grant support, Research Funding; Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights; Stemline Therapeutics: Research Funding; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding. DiNardo: Takeda: Honoraria; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; ImmuneOnc: Honoraria, Research Funding; AbbVie: Consultancy, Research Funding; Forma: Honoraria, Research Funding; Agios/Servier: Consultancy, Honoraria, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria; Foghorn: Honoraria, Research Funding; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding. Daver: Bristol Myers Squibb: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Sevier: Consultancy, Research Funding; Novartis: Consultancy; Astellas: Consultancy, Research Funding; Glycomimetics: Research Funding; Novimmune: Research Funding; Hanmi: Research Funding; Genentech: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; Gilead Sciences, Inc.: Consultancy, Research Funding; FATE Therapeutics: Research Funding; ImmunoGen: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Trovagene: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Other: Data Monitoring Committee member; Dava Oncology (Arog): Consultancy; Celgene: Consultancy; Syndax: Consultancy; Shattuck Labs: Consultancy; Agios: Consultancy; Kite Pharmaceuticals: Consultancy; SOBI: Consultancy; STAR Therapeutics: Consultancy; Karyopharm: Research Funding; Newave: Research Funding. Short: AstraZeneca: Consultancy; Jazz Pharmaceuticals: Consultancy; NGMBio: Consultancy; Novartis: Honoraria; Astellas: Research Funding; Takeda Oncology: Consultancy, Research Funding; Amgen: Consultancy, Honoraria. Issa: Kura Oncology: Consultancy, Research Funding; Syndax Pharmaceuticals: Research Funding; Novartis: Consultancy, Research Funding. Ravandi: Amgen: Honoraria, Research Funding; Xencor: Honoraria, Research Funding; Taiho: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Novartis: Honoraria; AstraZeneca: Honoraria; AbbVie: Honoraria, Research Funding; Prelude: Research Funding; Astex: Honoraria, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Syros Pharmaceuticals: Consultancy, Honoraria, Research Funding. Kantarjian: Ascentage: Research Funding; Astra Zeneca: Honoraria; Pfizer: Honoraria, Research Funding; Daiichi-Sankyo: Research Funding; KAHR Medical Ltd: Honoraria; Ipsen Pharmaceuticals: Honoraria; NOVA Research: Honoraria; Novartis: Honoraria, Research Funding; Immunogen: Research Funding; Astellas Health: Honoraria; BMS: Research Funding; Aptitude Health: Honoraria; Jazz: Research Funding; AbbVie: Honoraria, Research Funding; Precision Biosciences: Honoraria; Amgen: Honoraria, Research Funding; Taiho Pharmaceutical Canada: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal